How it works
Vertical Integration
Our company model oversees the entire tissue continuum, from Recovering amniotic tissue with our Labor and Delivery partner sites, to Processing and Manufacturing of the allografts, and finally Distribution to Hospital System’s Wound Care Centers and Operating Rooms for patient treatment.
This ensures the quality of clinical healing at a drastically reduced cost, offering more ACCESS to patients.






Pregnant Mother Enrolled in Donation Program

Mother Agrees to Donation and Successfully Completes FDA Donor Screening Assessment

Successful Cesarean Delivery of Healthy Baby in the United States

Recovery of Donated Placental Tissues
Microbiological and Serological Analysis of Donor Tissue

Donor Tissue Processing and Terminal Sterilization
Product Packaged for Shelf Stability


Pregnant Mother Enrolled in Donation Program

Mother Agrees to Donation and Successfully Completes FDA Donor Screening Assessment

Successful Cesarean Delivery of Healthy Baby in the United States

Recovery of Donated Placental Tissues

Microbiological and Serological Analysis of Donor Tissue



Donor Tissue Processing and Terminal Sterilization

Product Packaged for Shelf Stability


Pregnant Mother Enrolled in Donation Program

Mother Agrees to Donation and Successfully Completes FDA Donor Screening Assessment

Successful Cesarean Delivery of Healthy Baby in the United States

Recovery of Donated Placental Tissues

Microbiological and Serological Analysis of Donor Tissue


Donor Tissue Processing and Terminal Sterilization


Product Packaged for Shelf Stability
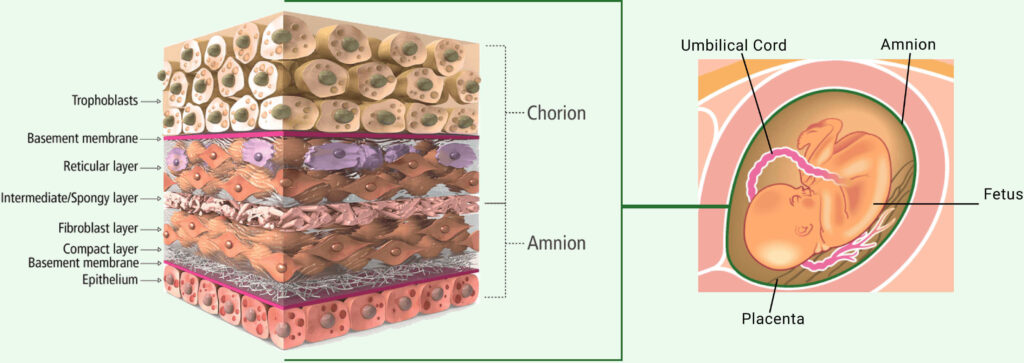
Amnion Regulation and Science
Amnion is Amnion
Do not be misguided by the “proprietary processing” marketing tactics companies use to claim their tissue products are superior in nature.
All amniotic allografts, no matter the manufacturer/supplier, have the exact same mechanism of action.
Amnion Is Amnion
Tissue Processing

Approved Tissue is Sent for Processing

Tissue is Dissected and Prepared for Washing

Tissue is Washed in Reagents

Tissue is Laid Out for Drying

Tissue is Cut and Pouched

Tissue is Sent for Terminal Sterilization

Tissue is Final Packager for Shelf Stability